
Traditionally, breast augmentation decisions were limited to a simple choice: saline or silicone. However, as aesthetic medicine continues to evolve rapidly, the conversation has shifted. Today, we evaluate more than just volume; we prioritize biocompatibility and rheology. In a competitive, cosmopolitan market like Los Angeles, where patients seek the perfect balance of safety and natural aesthetics, sixth-generation Motiva® implants have set a new benchmark for excellence.
At Moein Surgical Arts, we believe an educated patient is a satisfied patient. This guide explores the scientific realities of Motiva technology compared to established options like Mentor and Allergan, demonstrating why implant physics is just as critical to your outcome as surgical expertise.
Motiva breast implants have become a common specific request, particularly for women who prefer a softer, natural-appearing result, and have learned about “next-generation” implants. However, it would be more informative to consider Motiva implants not in the context of a trend, but purely as silicone-gel breast implants with specific characteristics, reviewed through the FDA’s PMA device approval process.
Additionally, in the USA, the FDA-approved products are Motiva SmoothSilk Round and Motiva SmoothSilk Round Ergonomix, which are used for breast augmentation (including revision augmentation) in females 22 years of age and older.
In this article, we examine scientific realities: where Motiva may vary significantly from past implant models, and where it does not. How a patient or a surgeon can make a safe, result-oriented decision will also be covered.
To explain why Motiva constitutes such an advance in science, it’s essential to understand what led to it. “Traditional” silicone breast implants (4th- and 5th-generation implants) emphasized “cohesivity,” meaning the ability to retain silicone if the “shell” broke. This resulted in the “Gummy Bear” breast implant, which was very cohesive.
Although these traditional implants were safe, the devices were criticized for being too “static.” They retained the same shape whether the female was standing, lying down, or exercising. Another area where traditional implants have fallen under scrutiny is the surface topography, often produced through salt loss or gas distension, and its interaction with the immune system.

The most dramatic change from the previous period occurs on the implant’s shell surface. Traditionally, surgeons used “textured” implants to prevent migration and reduce capsular contracture.
Older legacy brands tended to use macro-texturing (more aggressive, rougher surfaces). Yet, research later did associate such aggressive surfaces with chronic inflammation and, in some instances, BIA-ALCL (Breast Implant-Associated Anaplastic Large Cell Lymphoma).
Motiva took a different approach, using nanotechnology to deliver a “SmoothSilk” surface finish. The surface finish is not like conventional texturing and is designed to be “immune-blind.”
The Science: By creating a surface with controlled peaks and valleys at a microscopic level (less than 50 microns), Motiva implants minimize the friction between the implant and the surrounding tissue.
The Result: This biocompatibility reduces the body’s inflammatory response, which is the primary driver of Capsular Contracture.
Every type of silicone implant has a barrier to prevent “gel bleed,” when tiny silicone particles diffuse through the outer casing. In old-style implants, this barrier is transparent, making it impossible to inspect it directly.
Motiva has introduced the BluSeal indicator. The barrier layer is color-coded in light blue. If there is any imperfection in the shell’s manufacturing process or damage during handling, the light blue color may be affected. This serves as an added safety feature, ensuring that only a fully functional medical device is inserted into the body. This is one of the technological measures Moein Surgical Arts includes in its safety procedures.
Historically, if a patient lost their “implant card,” identifying the size, model, and serial number of an older implant required invasive imaging or even surgery.
Motiva solved this with Qid® technology, the world’s first FDA-cleared micro-transponder for breast implants. This tiny, battery-free RFID chip can be scanned externally, providing immediate access to the implant’s data. This is particularly useful for patients who may need a Breast Revision years later and have no record of their original surgery.
Peer-reviewed publications reporting 3-year clinical outcomes for Motiva (based on FDA-submitted study cohorts) describe low rates of several device-related complications during that time window, with high follow-up rates.
Two important limitations should be stated clearly:
A good consultation uses the published data as context—then returns to fundamentals: anatomy, surgical plan, and long-term monitoring.
In comparing Motiva to established brands, the information on the shell’s “tensile strength” is quite telling. Motiva shells are designed to be strong enough to be highly elastic. This enables “TrueFixation” and the use of smaller incisions, which significantly improve recovery after a breast augmentation procedure.
According to clinical studies, the rupture rate of Motiva’s 6th-generation implants is lower than 0.1%, which is well within acceptable levels, compared with the commonly stated 2-5% rupture rate for older studies involving 4th-generation silicone implants.
Patients often hope that a newer implant automatically means “no capsular contracture.” In practice, capsular contracture is influenced by:
The best approach is to discuss contracture as a system: device + technique + patient factors, then plan accordingly.
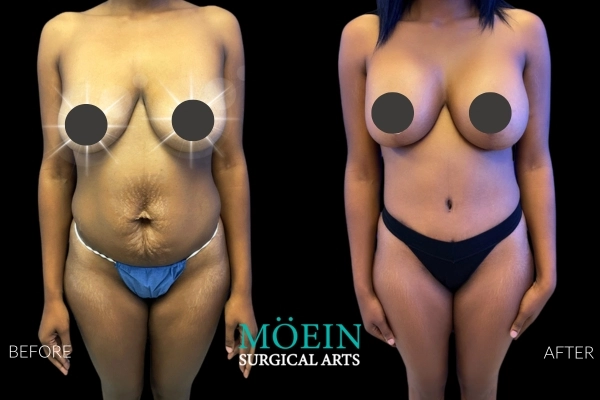
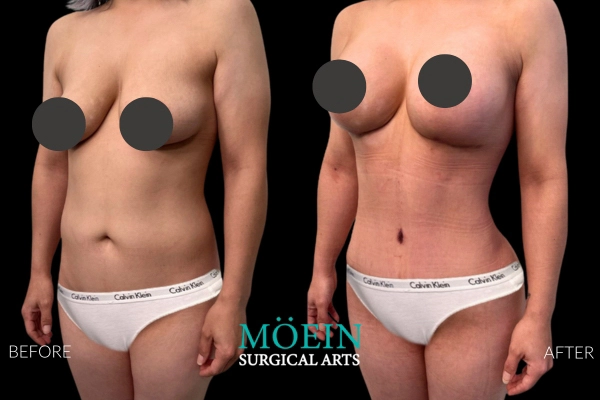
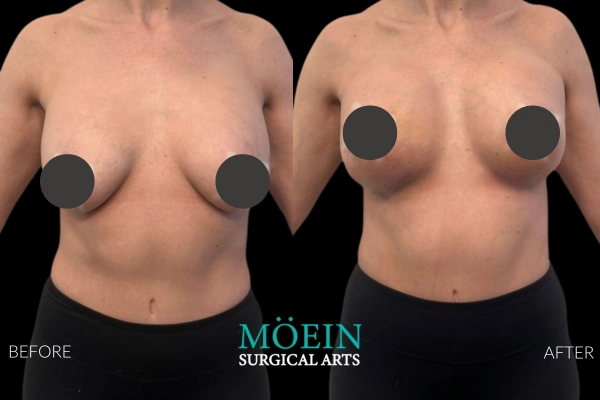
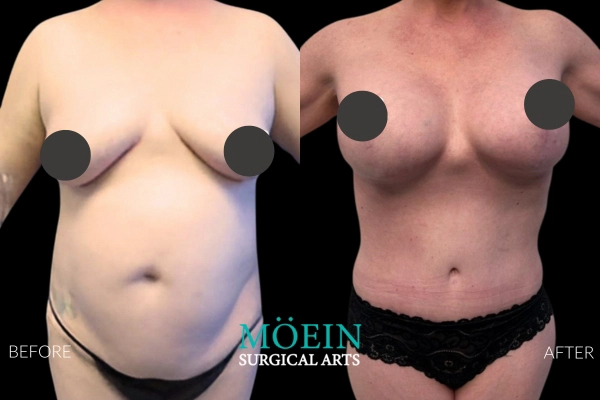
Many patients asking for “Motiva vs older brands” actually need clarity on whether their primary problem is:
A simple way to guide that decision is Breast Lift Vs Breast Augmentation, which clarifies when each option is appropriate and when combining them is the most honest path to the result you want.
For patients who need both, Breast Lift And Augmentation Before And After Photos can help set realistic expectations about what combination planning can achieve.
If your goal is subtle, proportionate breasts, “natural” is usually determined by:
For patients who want a visual reference, Natural Looking Breast Implants Before And After is a useful internal resource to frame realistic outcomes and shape expectations.
You can also review Breast Augmentation Before And After and Natural Breast Augmentation Before And After to compare implant-based augmentation with natural/implant-free approaches.
If we strip away marketing language, the fair conclusions are:
At Moein Surgical Arts in Los Angeles, Dr. Moein’s implant selection process is typically framed around measurable anatomy (base width, tissue thickness, skin quality), your aesthetic preferences, and a long-term monitoring plan aligned with FDA recommendations.
If you are considering Motiva Breast Implants or you are comparing Motiva Vs Traditional Implants—starting with a structured consultation is the most efficient way to determine which implant options truly fit your body and goals. Patients can begin with Virtual Consultation or use Contact Cosmetic Surgeon Los Angeles to request an in-person visit.
For patients who want to explore all options in one place first, Breast Procedures is a practical starting page that organises augmentation, lift, combined procedures, and related planning topics.